How Do You Teach Polar Vs. Nonpolar Molecules?
Have you heard someone explain polar molecules and not understood it? Yeah, sure, you understand the polar molecule part. But polar in the context of nonpolar starts to get more fuzzy. Then you look at an example problem, and you think, "Well I know that is nonpolar, but I can't tell specifically why, but my gut says 'Nonpolar!'".
You can use color and diagrams to show your students the difference for almost effortless understanding.
What is Polar?
There are two ways you can talk about polar molecules with your students: through “poles” or through electronegativity.
Most teachers will choose to use the “poles” explanation for beginner chemistry students or college prep courses. For more advanced chemistry students taking honors, AP, or IB chemistry, teachers will probably choose to use the electronegativity explanation.
Both are valid explanations, but the electronegativity explanation is a deeper (better) explanation that allows for more understanding, but can be overwhelming for beginning chemistry students.
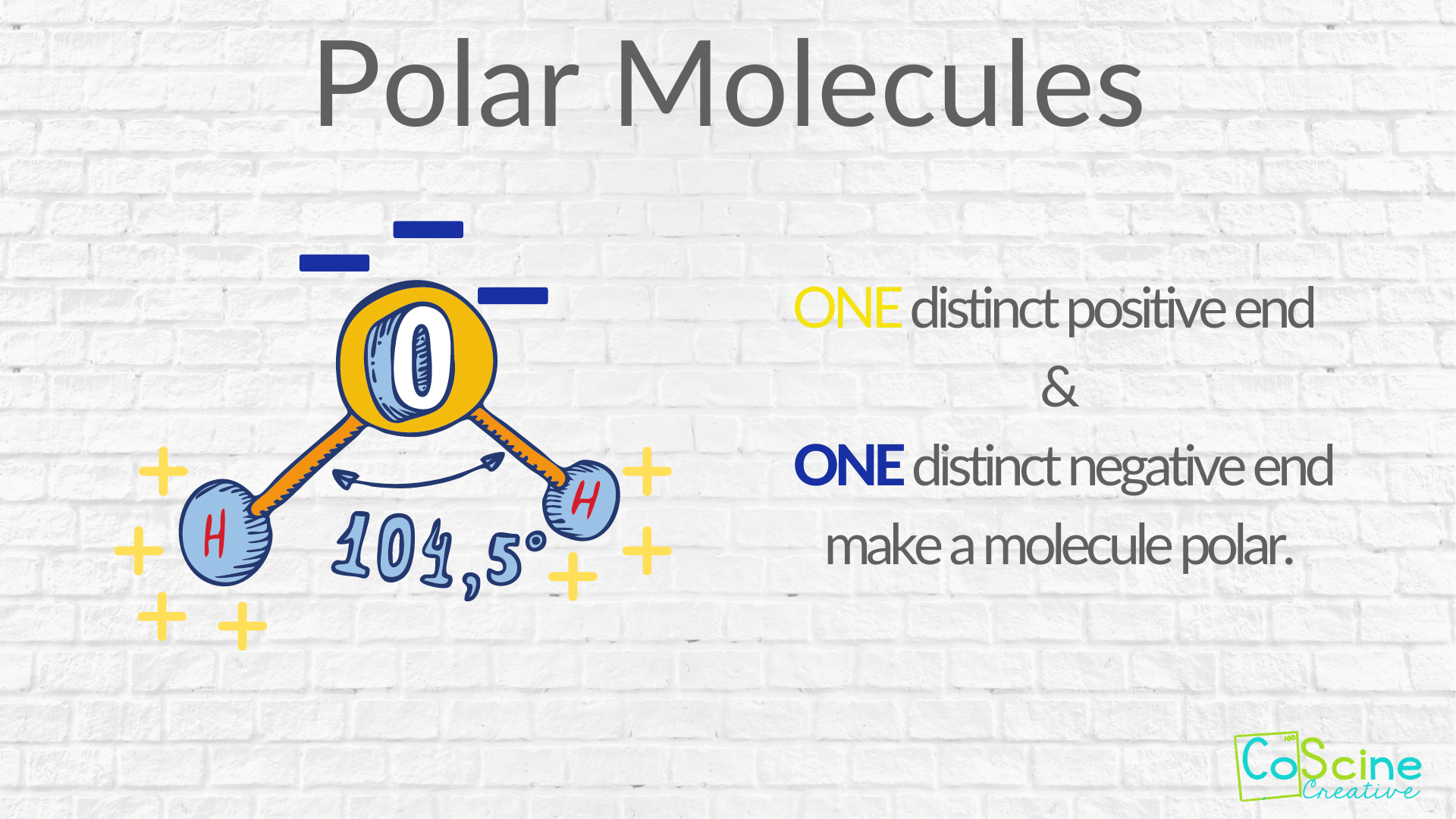
When introducing polarity through poles explain that there is one positive end and one negative end. Most teachers start out by drawing out a water molecule because it is very familiar to students. On the oxygen, draw out the electrons and put a minus sign to indicate that it has a partial negative charge. On the hydrogens, put a plus sign to indicate that they have partial positive charges.
It is that "polar opposite", where there is one end positive and one end negative that makes a molecule polar.
What is Nonpolar?
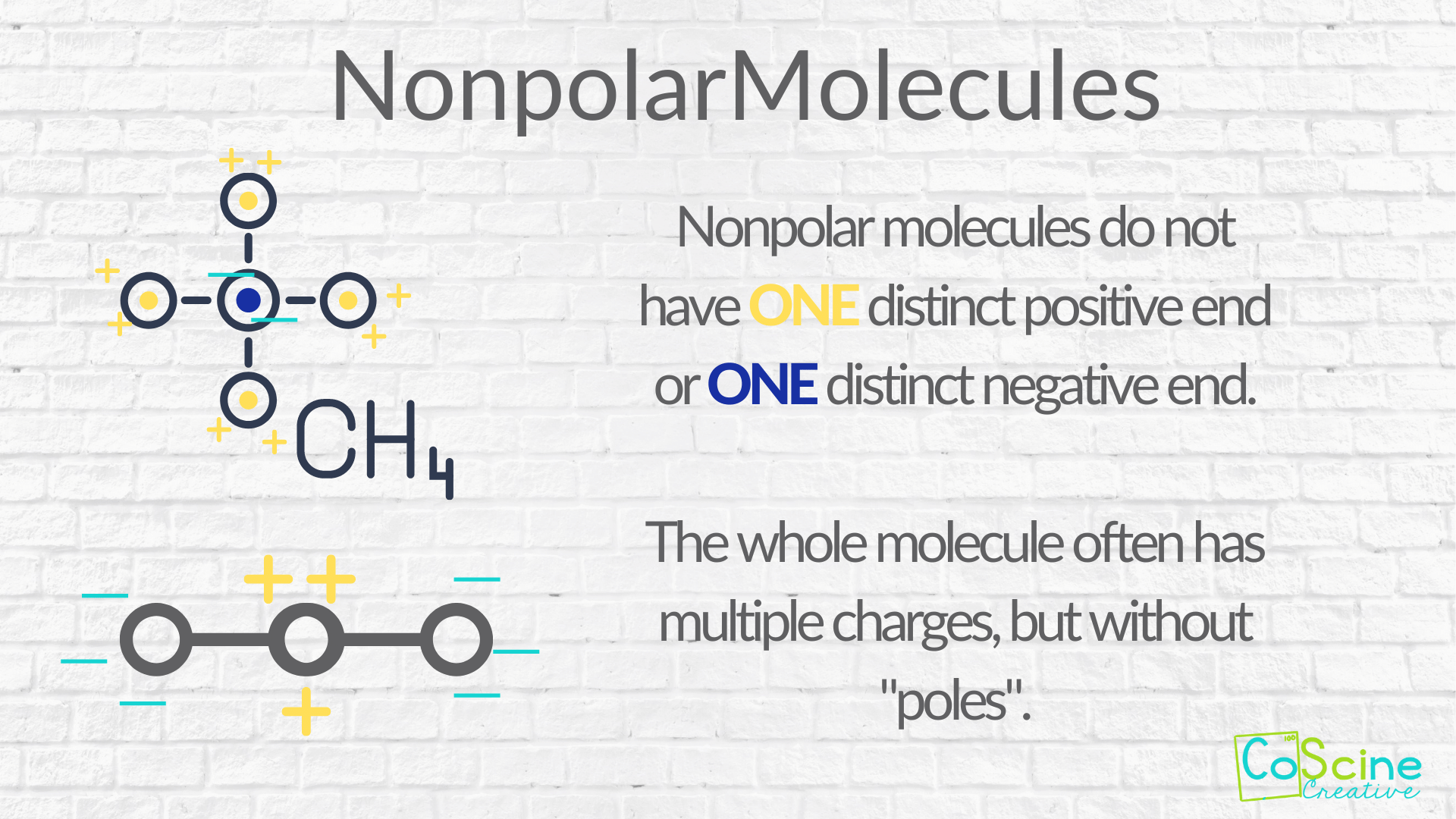
A nonpolar molecule is a molecule that doesn't have two distinct charge sites.
However, when you talk about this through the lens of electronegativity, you can see that there is some difference in charge throughout the molecule, but it is very small. That’s when many teachers use an electronegativity chart to determine degree of polarity.
Again, many teachers only discuss the electronegativty portion of this topic with their more advanced chemistry students.
Stop Saying "Cancel Out"
The one thing you can do to increase student understanding, is to stop saying "cancel out". When the phrase "cancel out" is used, the image that comes to mind is a left to right cancellation, or a top to bottom cancellation. But, often your students are thinking of the actual shapes of these molecules and it is hard to understand caddy-corner charges canceling each other out.
Instead, simply explain to students that polar molecules have one single positive end and one single negative end. Anything different should be labeled as nonpolar at the most basic level. Of course, if your students are more advanced, or mathematically inclined, have them use the electronegativity chart.
Want the Diagrams for Your Class?
Check out the doodle notes here.