What is the Difference in a Shell, Subshell and Orbital?
Nothing is more confusing to students than the difference in a shell, subshell, and an orbital. Except for assigning quantum numbers to an electron.
But that is a topic for another article.
However, let’s learn to explain the difference in a shell, subshell, and an orbital without breaking a sweat.
A Shell is the Coefficient Number
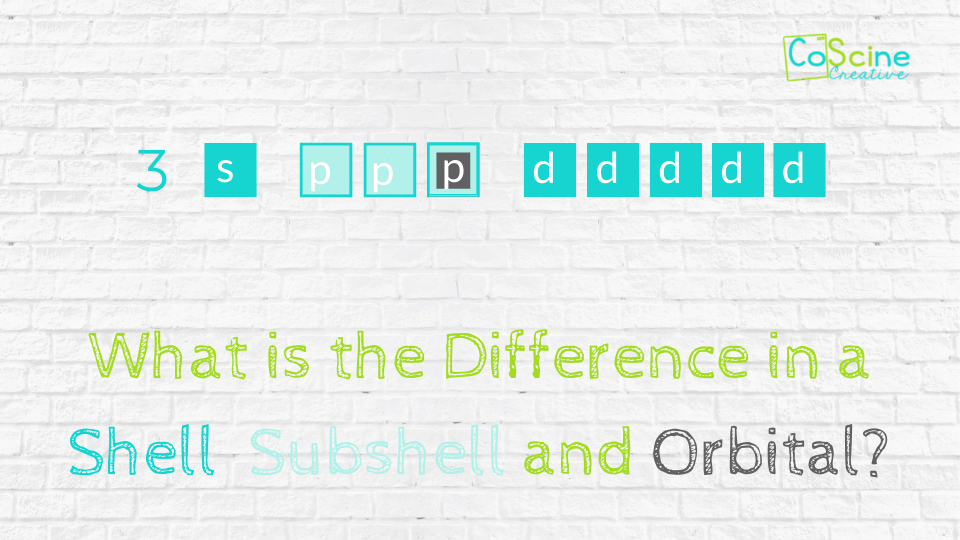
A shell is all the places an electron could be within one energy level. For example, all the electrons in the 4 shell are 4s, 4p, 4d, and 4f. Those would encompass the 4 shell. Knowing that makes understanding shells a lot easier right? It’s just everything under one energy level.
Always start with shells when teaching this to students. Because if they can understand a shell, they can understand a subshell.
A Subshell is One Letter Group within a Shell
If we look at the example above, there are 4 subshells within the 4 shell: 4s, 4p, 4d, and 4f.
Students readily understand this concept, but need to take notes and practice identifying shells, and subshells to internalize the concept.
For practice, consider using these doodle notes and this worksheet page. If you are looking for more resources and worksheets on this topic, I have another blog post here.
Orbitals Hold 2 Electrons
Sometimes we misuse the term “orbital” since it is so elusive. Show the word orbital refers to the individual space that 2 electrons occupy.
A lot of the time we say “s orbital” and that is correct because there is only one orbital in the s subshell. But, when we shift to saying p orbital, most of the time it isn’t correct, because there are 3 p orbitals and we are not talking about an individual orbital, but the subshell.
I’ve done this myself many times which is why I created this worksheet and the 3rd page on my quantum doodle notes.
In short, an orbital can only be a single block or line where 2 electrons can live.
Quantum Chemistry Simplified
Hopefully that made everything super simple so you have a concise way to explain this area of quantum chemistry to your students.
Knowing how to explain this better is going to make you the best chemistry teacher ever. Check out this worksheet and doodle note packet that goes along with this list.
Worksheets Mentioned on this Page: